inprocess as per usp ip bp tablets

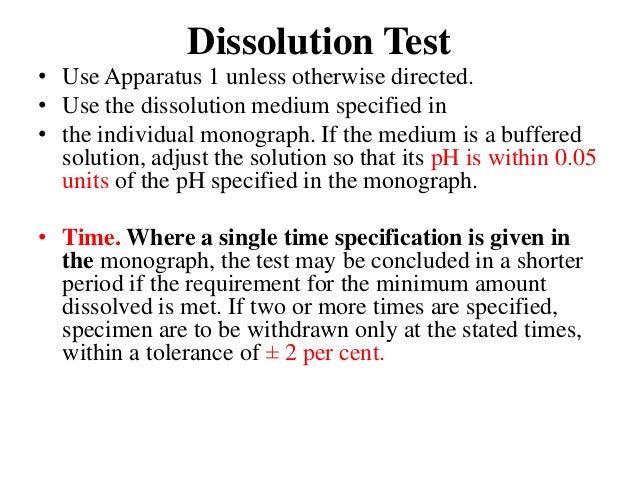
disintegration test dissolution test
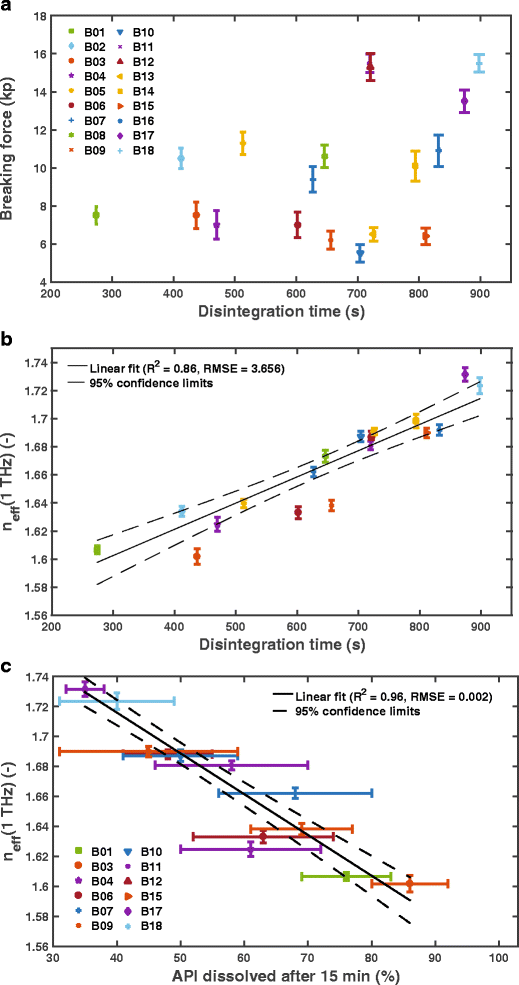
disintegration time and dissolution

cyberleninka

factors affecting disintegration

table 1 from fast disintegrating
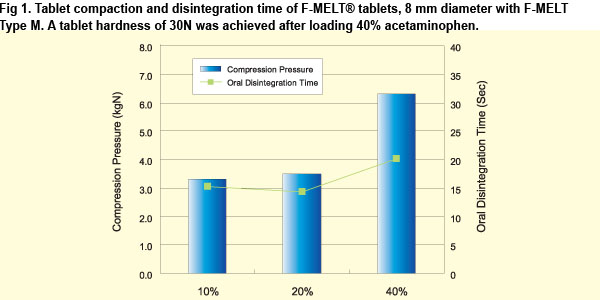
fast disintegrating oral tablets
quality control tests of tablet
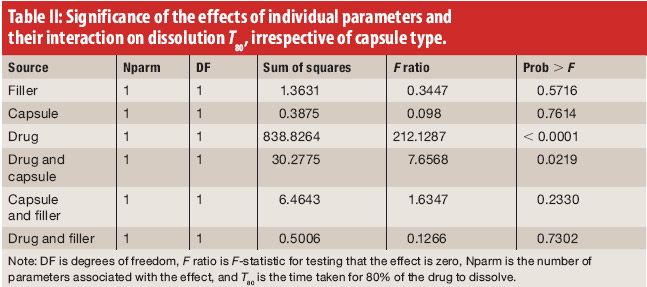
the effect of overencapsulation on
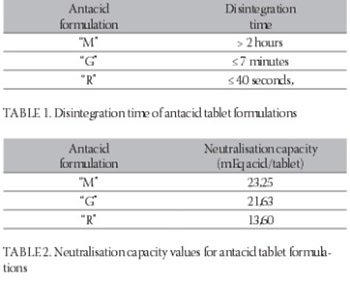
neutralisation capacity of antacids

requirement of disintegration time pharmaceutical tablets

disintegration time of tablets

usp 32 disintegration method and
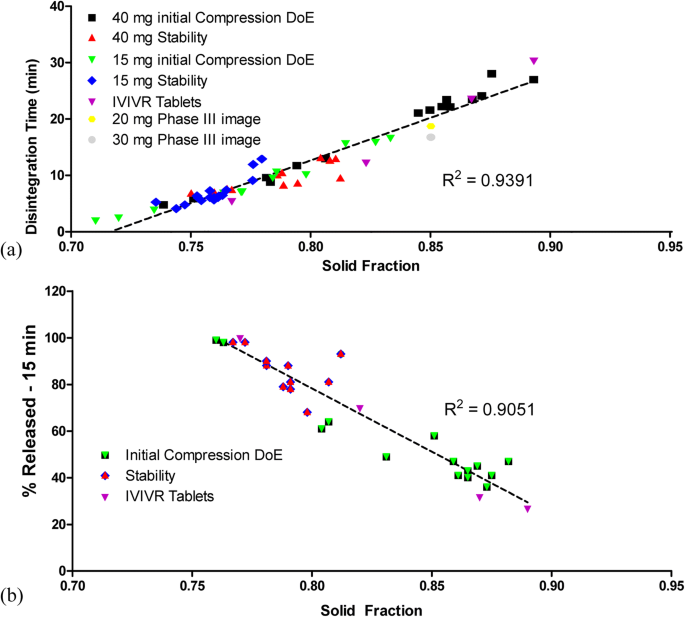
predicting in vitro dissolution

pdf pharmaceutical evaluation of
.jpg?h=567&w=700&hash=C8F5662E10389193402211FD3BE3F5FEE3379994&hash=C8F5662E10389193402211FD3BE3F5FEE3379994&la=en)
why oral disintegrating tablets

disintegration time of tablets produced

scielo

hnmrhq4xxebfnm

1library

fast disintegrating tablets
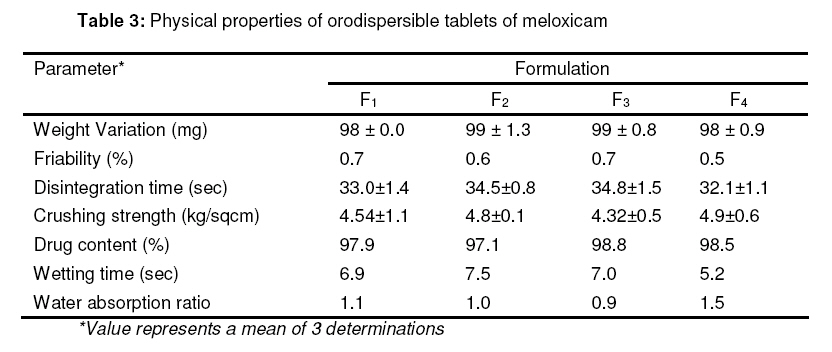
bioline international official site site up dated regularly

enteric coated tablets
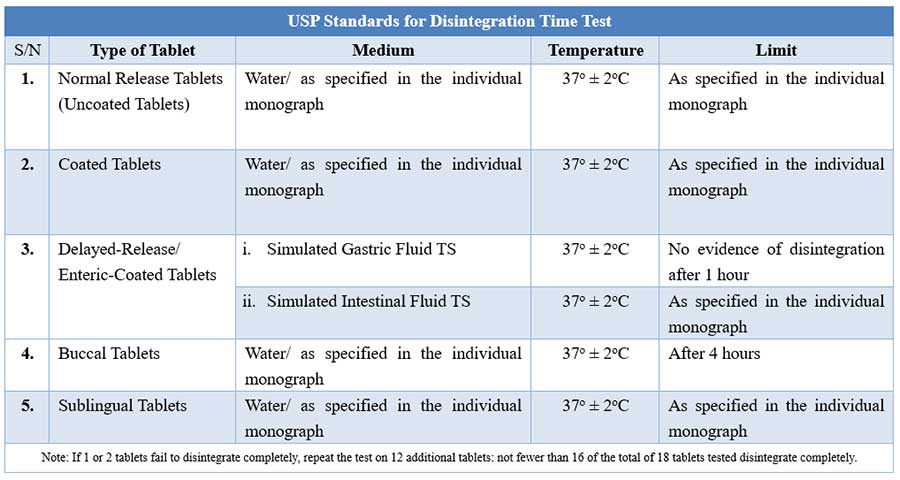
quality control tests for tablets

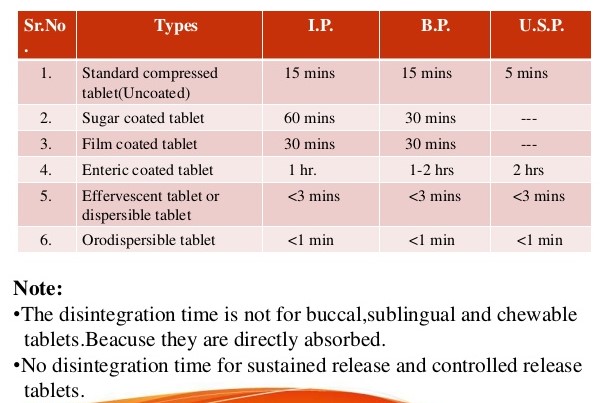
usp limits for disintegration time of
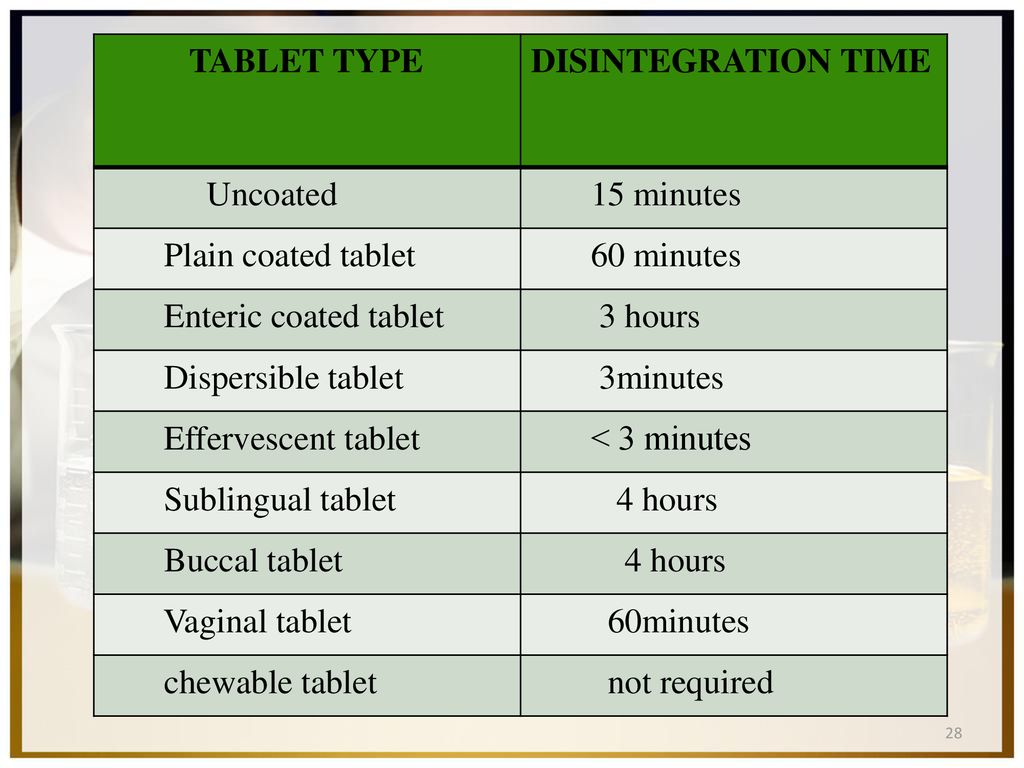
بورجوندي سفينة فضائية أخوة disintegration time of coated tablets

ep0172014b1 formulations of ibuprofen

dissolution profile of pcm dc tablet

disintegration test and apparatus
You May Like

