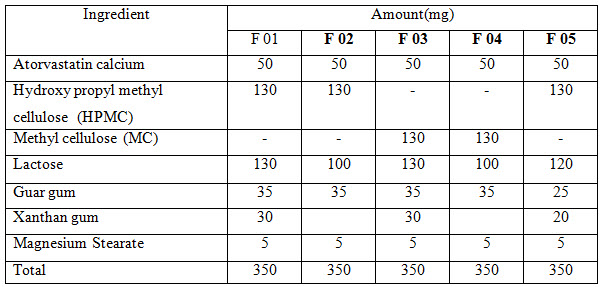
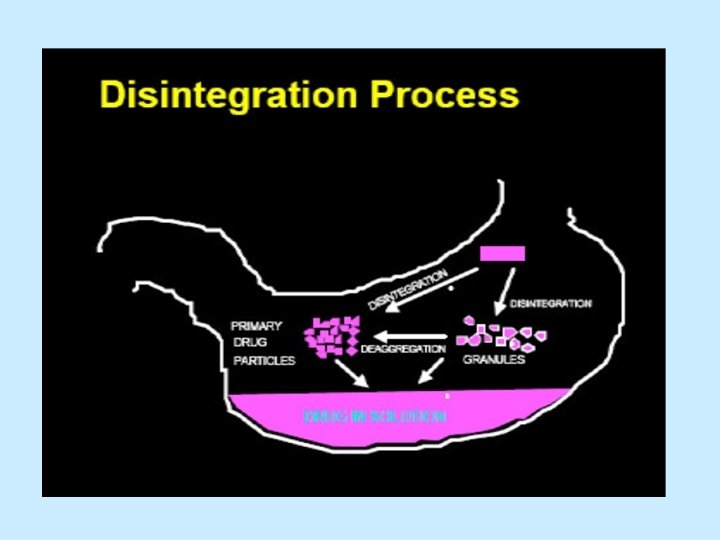
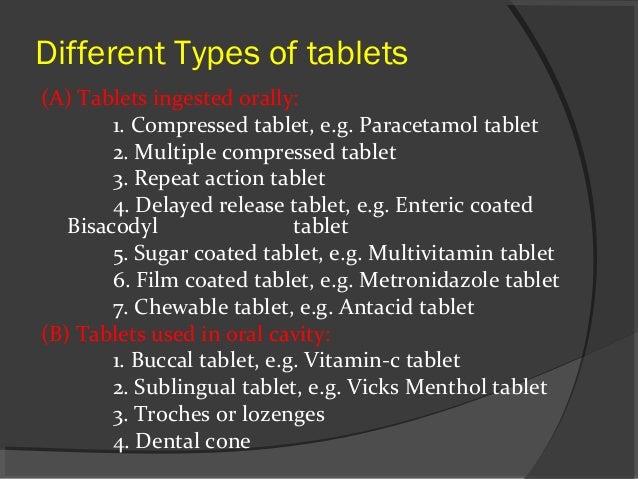
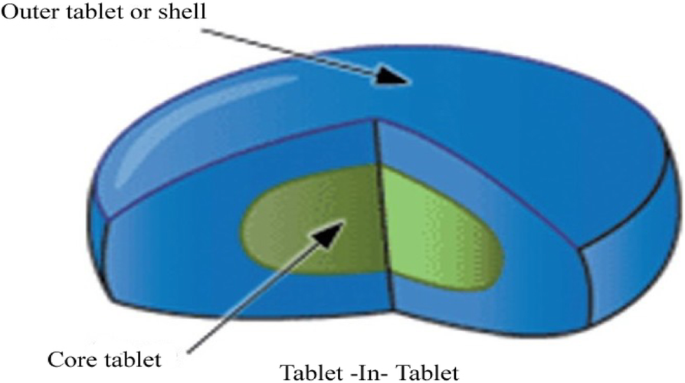
tablet in tablet formulation
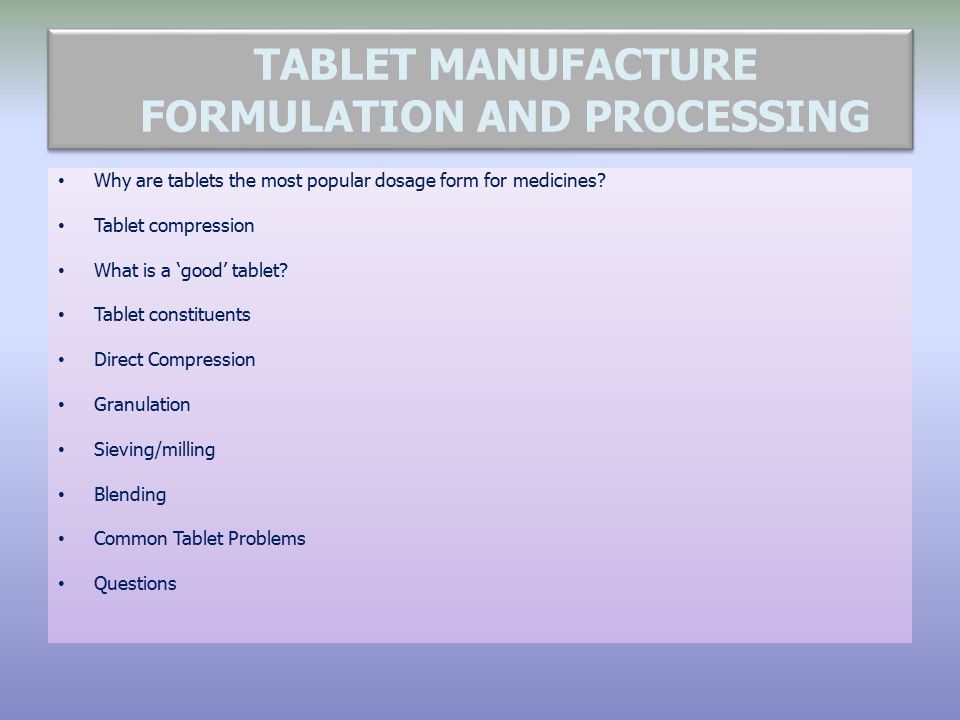
Either because the tablet formulation is too brittle or not adhesive enough or because the powder being fed to the tablet press contains too much air has too low bulk. Today s guide explores all the intricate details of tablet manufacturing. Formulation studies then consider such factors as particle size polymorphism ph and solubility as all of these can influence bioavailability and hence the activity of a drug.
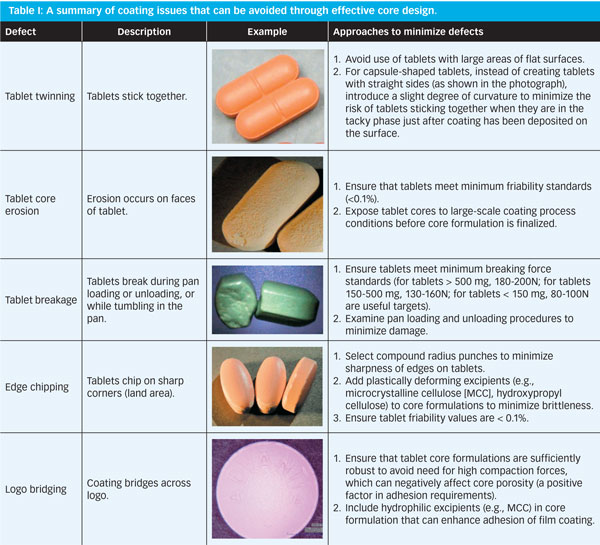
To tolerate attrition of tablets during coating process they must be resistant to abrasion and chipping.

Tablet in tablet formulation. Binding is tablet defect where a tablet adheres seize or tear in the die or a film is formed on the die area and hindered the ejection of the tablet or splits during the molding pressing process. This enables you to enter ingredients and work out how much of each ingredient you need to make a batch. This is caused by air being compressed with the tablet formulation and then expanding when the punch is released. The icu tablet counter provides broken tablet detection capabilities.
Tablet mix calculator free tool to help pill formulation. As the tablet surfaces that are brittle and soften in presence of heat or effected by coating composition and tend to become rough in the early stages of coating. Apart from capsules tablets is a popular oral dosage form a reason you need to understand the tablet manufacturing process. Causes and remedies of binding related to formulation.
Trimethoprim is metabolized in vitro to 11 different metabolites of which five are glutathione adducts and six are oxidative metabolites including the major metabolites. The drug must be combined with inactive ingredients by a method that ensures that the quantity of drug present is consistent in each dosage unit e g. Tablet definition a number of sheets of writing paper business forms etc fastened together at the edge. The formulation of n 4 hydroxy metabolite is mediated via cyp2c9.
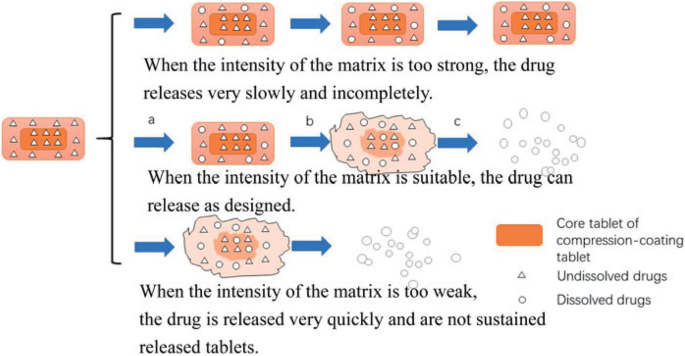
If this breaks the tablet apart it can be due to incorrect machine settings or due to incorrect formulation. A second coating of course can be applied once the first is dried to ensure that the core is effectively sealed. Tablet tablet double strength. The choice of sealant formulation is influenced by the porosity of the tablet since highly porous materials would soak up the sealant solution preventing the creation of a uniform coating over the whole tablet.
Knowledge on tablet manufacturing process is important for students studying pharmacy physician or medical practitioners tablet press manufacturers among other professions. The machine can detect broken pieces as small as 0 5 mm up to 30 broken piece. Tablet properties tablet to be coated must posses the proper physical characteristics like spherical shape and uniform surface.






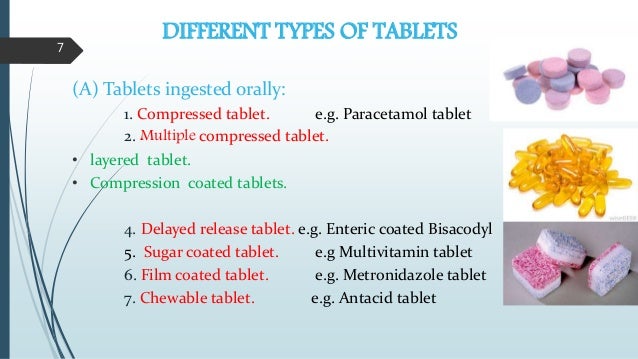

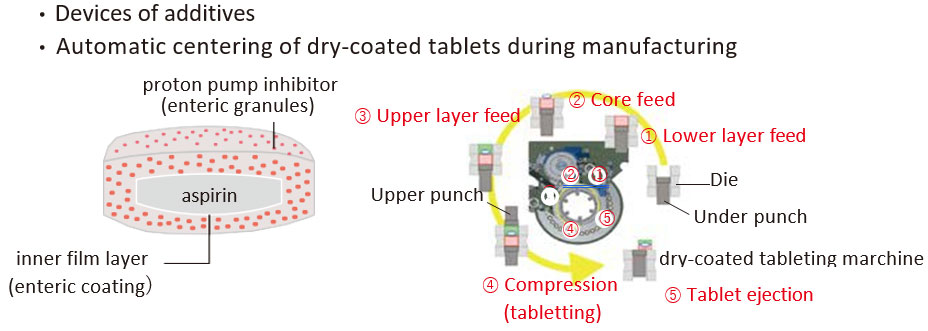
































































.jpg?h=271&w=400&la=en&hash=F75D9E98B516865F346CF3E3BF38337FE99C245F)